New medical treatments, including drugs and medical devices, cannot be approved for patient use until they undergo clinical trial testing. This testing uses volunteers to assess the safety and effectiveness of the treatments.
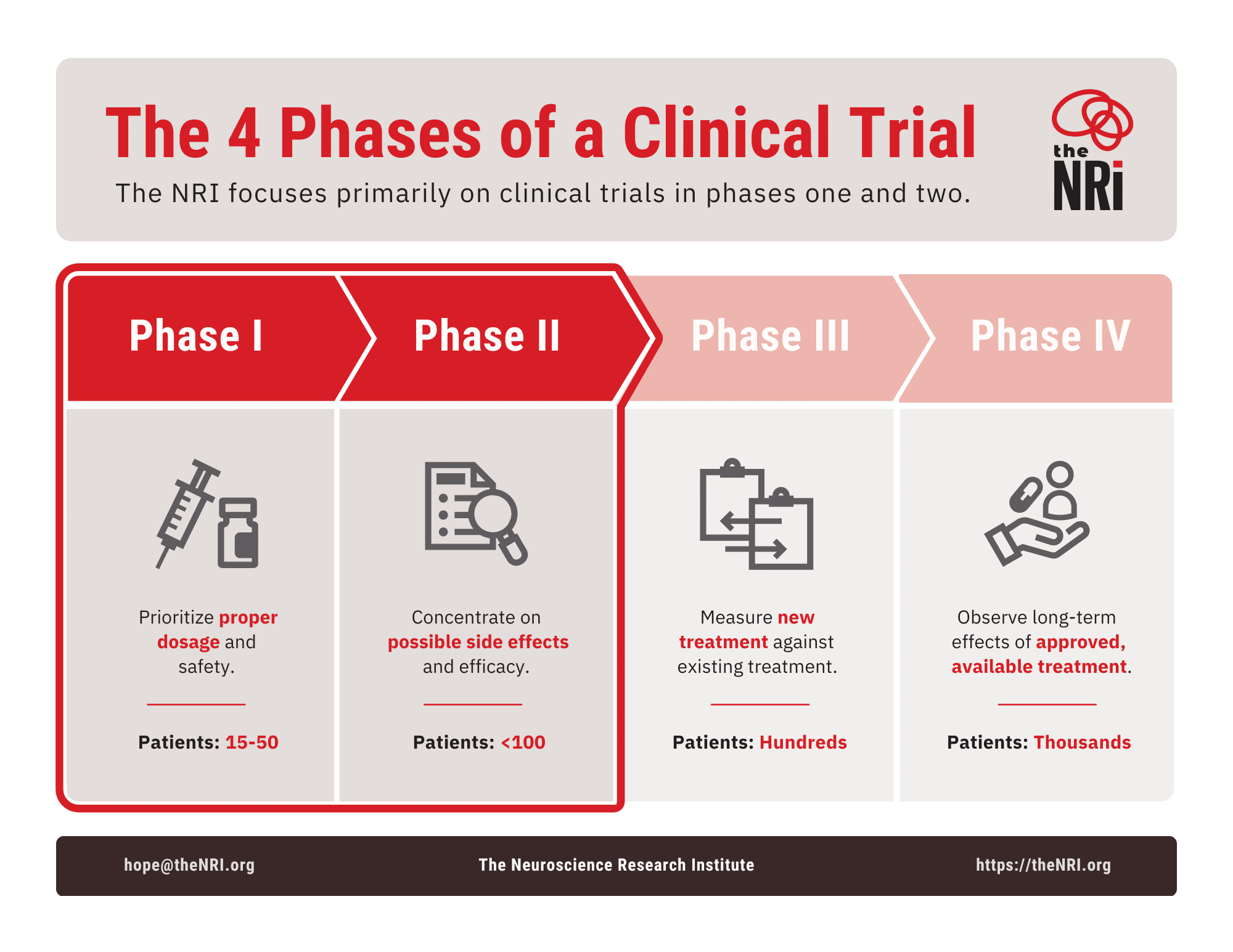
Most clinical trials consist of four key phases that new treatments must go through before they can be submitted for approval. Each phase involves more participants and addresses important questions. This helps researchers decide if the treatment is ready to be reviewed by regulatory agencies. These agencies then determine if the treatment can be approved for patient use.
What are these four phases?